RD-Biotech offers a complete range of custom services with its Antibody Engineering platform.By contacting RD-Biotech you will:

An integrated platform for the development of your therapeutic antibodies: from target to candidate
Bioproduction
RD-Biotech is a service company recognized for its know-how and experience in the field of antibodies. In particular, the company offers multi-technology services for the development of antibodies for R&D, diagnostic and therapeutic purposes.
Production of your candidates according to an adapted and personalized strategy according to the biological tools and the available data:
- The target (proteins, peptide, protein sequence, bibliographic data)
- The murine monoclonal antibody secreting clone
- Nucleotide sequences of interest (VH and VL sequences)
- Expression vectors ready-to-use for transfection
- The cell lines
Depending on the progress level of your project, RD-Biotech has the expertise and the technological platforms to:
- Express your target (vector construction and antigen expression)
- Develop monoclonal antibodies (Hybridomas and phage display)
- Sequence monoclonal antibodies
- Develop and produce your recombinant antibodies
- Recombinant mice or other species, different isotypes possible
- Chimerization of your candidate
- Humanization of your candidate
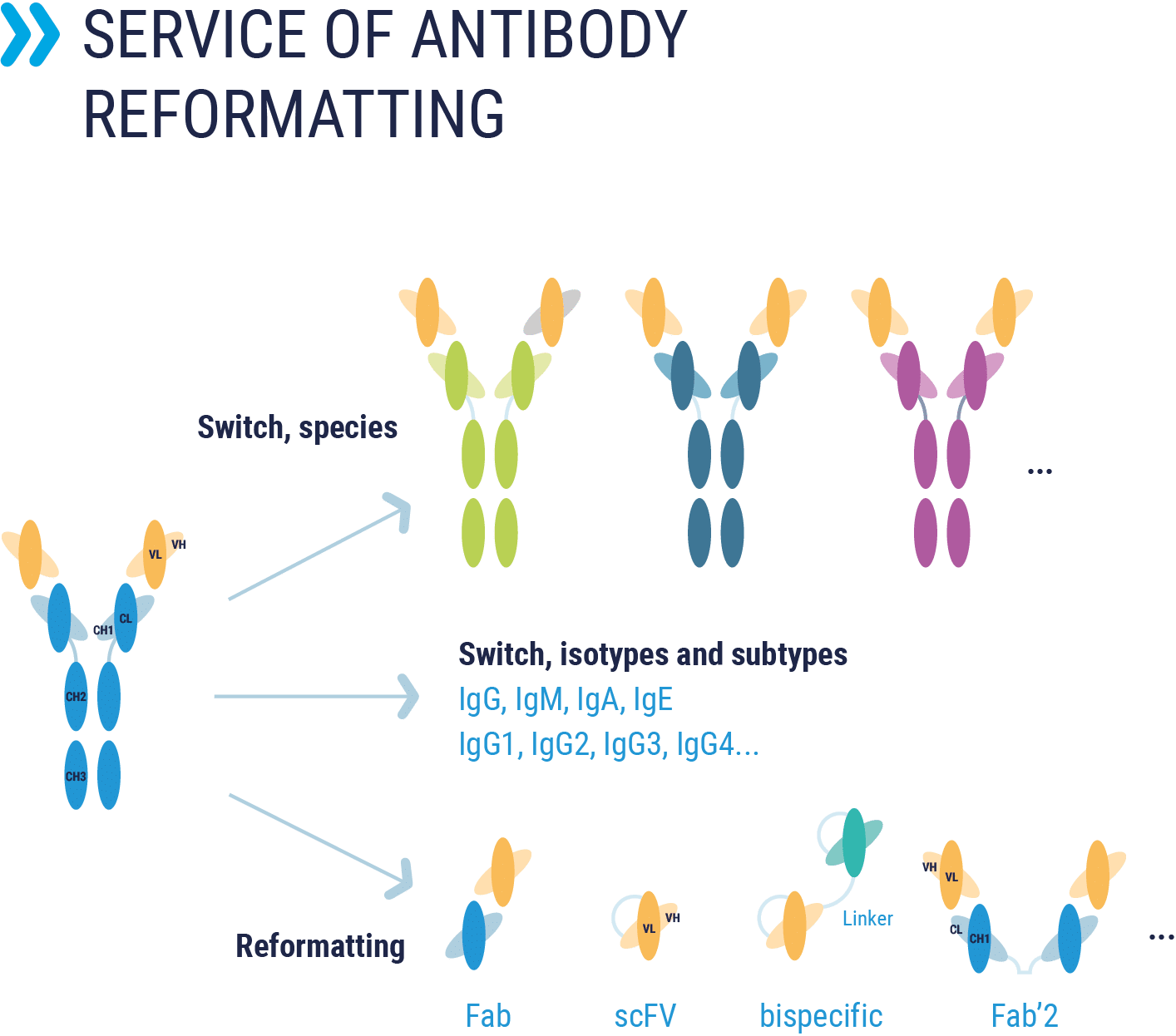
- Genetic modifications: Reformatting with another species, change of isotypes, antibody fragments (Fab, scFv, nanobodies), bispecific format, …
- Production system optimized in mammalian cells: CHO cells, HEK cells (other cells – contact us)
- Transfection from vectors optimized for mammalian cells
- R&D “endofree” quality or High Quality Grade
- Reproducibility: in vitro procedure, chemically defined medium, for excellent inter-batch reproducibility
- Purity: serum free mammalian cell expression system eliminating the risk of contamination by serum components, and providing a level of purity greater than 95%.
- Capacity: pilot batch production or large-scale production (up to several hundred mgs)
- Speed: high-performance expression system allowing the supply of batches of recombinant antibodies in a very short time: Supply of a batch of 100 mg of recombinant antibodies purified and checked in less than 3 weeks.
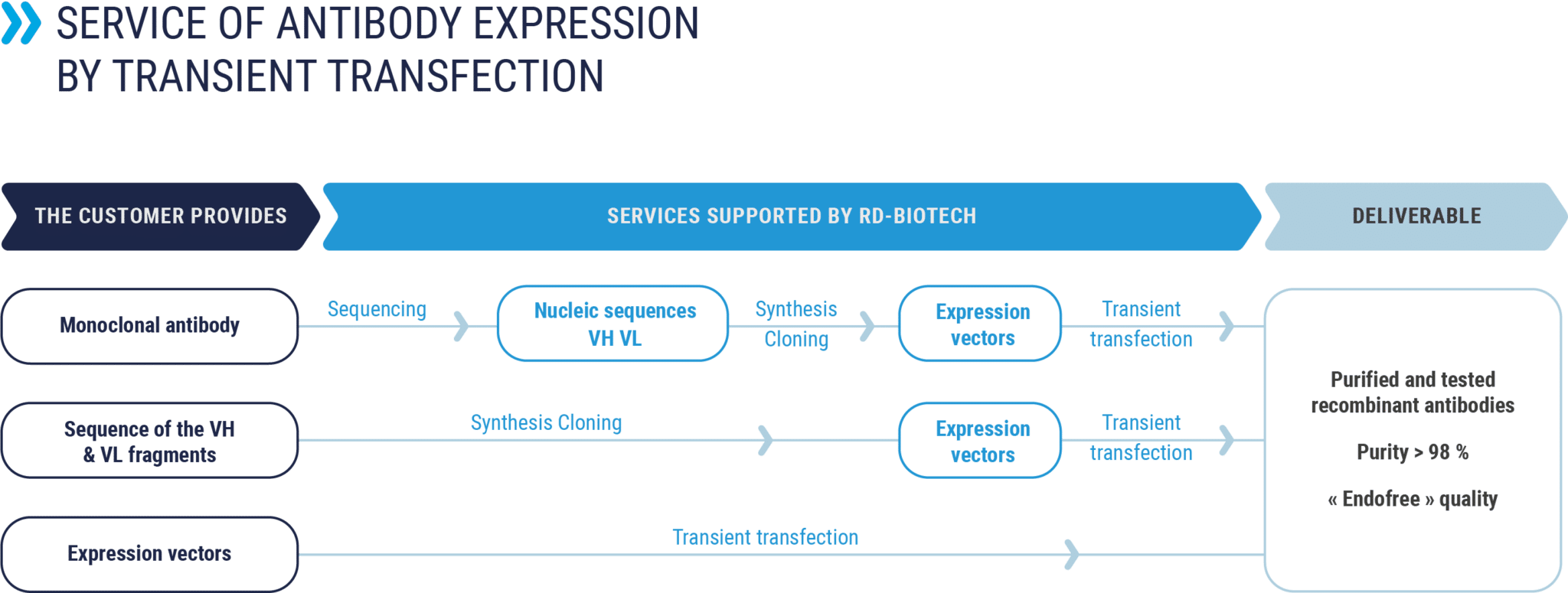
Antibody production by transient transfection
CHO, HEK293 or other cells
The standard procedure includes the following steps (certain steps may be carried out by the customer, to be specified)
- Optional step: monoclonal antibody sequencing, heavy and light chains
- 1st step : synthesis of variable fragments VH and VL
- 2nd step : subcloning the fragments into the expression vector. Quality controls.
- 3rd step : antibody expression pilot by transient transfection.
- 4th step : production monitoring by ELISA (Discover FastELISA)
- 5th step :purification of the pilot by affinity chromatography (Protein A support)
- 6th step : Quality controls(analytical services)
- Purity control by SDS-PAGE gel electrophoresis (reducing and non-reducing conditions)
- Determination of total proteins by absorbance measurement (spectrophotometer)
- Other controls possible, consult us
- 7th step : Packaging and shipment of the mAb, accompanied by report.
Deadlines :
Step 2: 2 to 3 weeks
Steps 3 to 5: approximately 3 weeks
For a Fastrack service: contact us!


Go No Go: Large Scale production and purification (up to gram scale) according to the validated procedure.
Optionally, the antibodies produced in vitro can be purified under “Endofree” conditions, which requires the use of non-pyrogenic buffers and containers.
After the affinity chromatography step, the purity of the antibody can be improved by different methods:
- by a preparative SEC step which will eliminate the aggregates;
- by an ion exchange chromatography step, which also removes any endotoxins that may be present. The monoclonal antibodies produced in vitro by RD-Biotech under “Endofree” conditions have an endotoxin level of less than 1 EU / mg.
Additional quality controls can be added to the basic controls, depending on the specifications of the ordering customer and the applications:
- Mycoplasma test
- LAL test to determine the level of bacterial endotoxins. The LAL (Limulus Amebocyte Lysate) test is performed using a regulatory kit according to the supplier’s protocol.
- Bacterial and fungal contamination test
- Control by analytical SEC
- Desalting of antibodies
- Control of specificity, biological activity … (ELISA, Western Blotting, Octet, Cytometry, custom bioassays …)
- Protein A residue determination
- Determination of bovine Ig contaminants (RD-Biotech FastELISA Kit)
Discover FastELISA - As part of the High Quality Grade process, specific controls are applied
Contact us
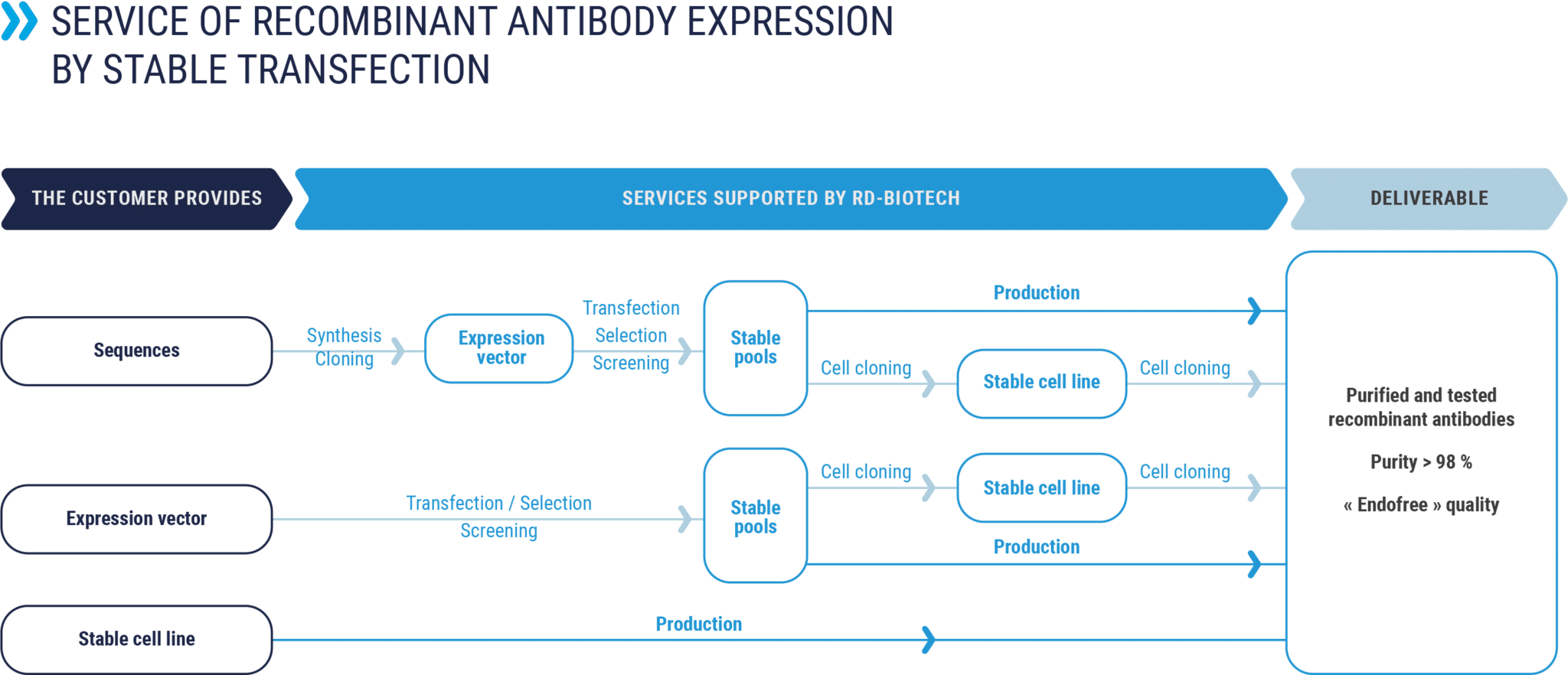
Production by stable transfection
Production of recombinant antibodies from a cell line or a stable pool of cells, supplied by the ordering customer or developed by RD-Biotech
Custom contract services
Recombinant antibodies have undeniable advantages because they are produced according to an animal free process, in a chemically and biologically defined environment, unlike hybridomas.
RD-Biotech puts its expertise and know-how in molecular & cell engineering at the service of its customers to modify recombinant antibodies and generate variants according to your specifications.
- Success rate> 98%
- Scale-up of up to several hundred mg
- Up to 50 variants
- Royalty-free
Benefits :
- Purity: can reach very high levels (> 98%) by expression in mammalian cells and without serum, eliminating the risk of contamination by serum components
- Reduction of production times
- Process reproducibility
- Antibody engineering
Antibody sequencing
RD-Biotech can carry out the heavy and light chain sequencing of the monoclonal antibody candidate
Antibody reformatting
Reformatting service for your antibodies: scFv, Fab fragments, bispecific, change of isotypes or sub-isotypes …
Chimerization
Chimeric antibodies are formed by the association of the variable regions of a “parental” or “donor” antibody with the constant regions of an “acceptor” antibody (of a different species). The combination of murine variable domains and human constant domains is the most common, but other combinations are also possible.
Chimerization is a simple and straightforward process for retaining the affinity and specificity of the parental antibody.
- Development of the parental monoclonal antibody (if requested)
- Sequencing of the VH and VL variable fragments of the parental antibody (if requested)
- Construction of expression vectors: codon optimization, selection of constant domains (species and isotype), cloning of VH and VL variable domains. Quality controls.
Discover molecular biology - Co-transfection and production of chimeric antibodies by transient transfection in CHO cells
Discover recombinant antibodies production - Productivity evaluation by ELISA (FastELISA kits)
Discover FastELISA - Purification by affinity chromatography (protein resin A, G, L, etc.)
- Quality controls:
(analytical services)- Purity control by SDS-PAGE gel electrophoresis (reducing and non-reducing conditions)
- Determination of total proteins by absorbance measurement (spectrophotometer)
- Additional controls offered (depending on the project)
- SEC HPLC
- Determination of affinity
- Functional tests
- Other tests available
Humanization
Humanized antibodies constitute the majority of therapeutic antibodies approved on the market.
The humanization of a parental antibody (eg mouse) is carried out according to a modernized version of “CDR-grafting” according to the approach invented by Winter et al. (“Medical Research Council” – Cambridge – UK). The principle of this method is to reformat a human antibody (germline sequences) so that it contains the CDRs (Complementary Determinant Regions) of the parental antibody in order to reduce its immunogenicity in humans. Humanization by “CDR-grafting” requires that mouse antibody residues involved in antigen recognition be preserved in the humanized version of the antibody.
Following the humanization step, sequence optimization may be offered with optional additional analyses by molecular modelling:
The objective being:
- An increase in manufacturability (reduction of heterogeneity)
- A possible increase in affinity and biological activity (IC50)
The optimized procedure proposed by RD-Biotech includes the steps described below:
Humanization by CDR-grafting:
- Identification of framework regions (FRs) and CDRs of the parental antibody.
- Search and alignment of parental antibody sequences with the most homologous human germ sequences
- Identification of critical positions.
- Selection of a germline sequence for each chain (VH and VL) based on sequence analysis and identification of critical positions
- Assessment of the suitability of the substitution at each diverging position between the murine and human sequences based on structural analysis.
- CDR-grafting by analysing sequence differences between parental sequences and acceptor sequences of the antibody.
Sequence optimization:
- Improving the manufacturability of the antibody and optimizing its affinity and activity.
- Provision of an interim report including strategy, aa sequences of VL and VH of humanized variants to be synthesized and tested as well as suggestions for sequence optimization.
For each construction, the stages of construction of the expression vectors, co-transfection, production and purification are carried out, according to the same procedures as those described in the previous chapter “production by transient transfection”
Quality controls include:
- Purity control by SDS-PAGE gel electrophoresis (reducing and non-reducing conditions)
- Determination of total proteins by absorbance measurement (spectrophotometer)
- Additional controls offered (depending on the project)
- SEC HPLC
- Determination of affinity
- Functional tests (bioassay platform)
- Other tests available, contact us
At the end of this step, a report is issued, including, for each candidate, the strategy followed, the results obtained and certificates of analysis.
From an identified “lead candidate”, a second humanization cycle can be proposed in order to substitute any murine residues with their human counterparts in the “non-CDR” regions and thus increase the human % in VH and VL
- Sequence optimization to eliminate potential PTMs present in CDRs
- Complementary analysis by modelling
These different optimization approaches can be carried out in parallel.
Characterization and validation of candidates
specificity, affinity, bioactivity, ...
RD-Biotech’s analytical platform offers a wide range of services:
- Concentration, Purity
- Determination of endotoxins levels
- Determination of the aggregate rate
- Determination of the residual rate of contaminants: proteins A,….
- Sequencing of the variable parts VH and VL
- Analysis of the specificity of recombinant antibodies: ELISA, Western Blot, etc.
- Analysis of the affinity of recombinant antibodies: determination of Kd, Kon, Koff using Octet technology
- Stability analysis: DSC
- Analysis of the biological activity of antibodies: wide range of bioassays (ADCC, ADCP, CDC, … consult us) and custom development service
Phage display
Thanks to the know-how of the mAbexperts team, RD-Biotech offers the development of recombinant antibodies using Phage Display technology:
Phage display in mice:
- Excellent alternative to hybridoma technology
- Fast process (3 to 5 months)
- Provision of naive or immune banks (fab, scFv)
Phage display in llamas:
Development of your VHH by immunization of llamas and using phage display technology.
Other services
Our sales team as well as our scientific and technical experts are at your disposal to offer you the service adapted to your request!