RD-Biotech’s molecular biology platform offers a complete range of custom services.By contacting RD-Biotech, you will:

Bioproduction
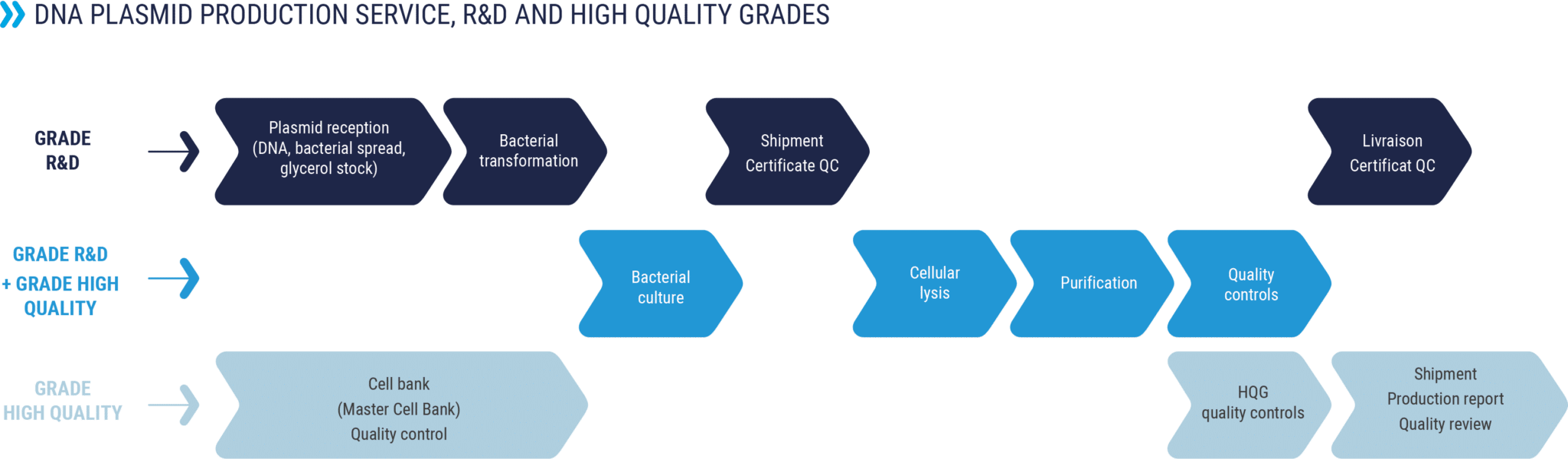
DNA PLASMID PRODUCTION
With more than 1500 plasmids produced each year, RD-Biotech has developed unrivalled expertise in the production of plasmids, for research applications up to clinical studies.
Whether you require 1 mg of DNA for an R&D study or 500 mg for in vivo applications, our goal is to meet your demands, in terms of quantity, quality and deadline.
A GMP-Grade service: In order to complete its pDNA biomanufacturing platform, RD-Biotech now offers GMP Quality plasmid DNA, an essential raw material for new therapies: mRNA/DNA based therapies, Cell therapies, virus-based therapies, …
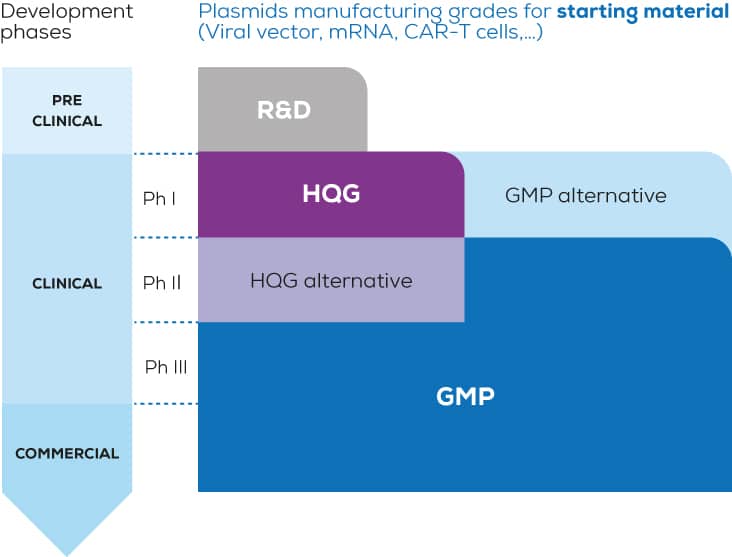
“Endofree” Research & Development grade plasmid production
Plasmid DNA for your R&D and preclinical applications
RD-Biotech is capable of supplying batches of 10 mg in 3 days, batches of 100 mg in only 6 days!
- “Endofree” quality: we guarantee a level of residual endotoxins of less than 0.1 EU / µg of DNA
- High production capacity: from a few milligrams up to several grammes per plasmid. QUANTITY GUARANTEED!
- Very short deadlines
- Production of plasmids from DNA, bacterial spreads or glycerol stocks
- Pre-production controls according to the reference restriction profile: selection of the clone to be amplified, analysis of the restriction profile, quantification, etc.
- Final quality controls included, without additional cost: Quantification by spectrophotometry – Control by enzymatic digestion.
- >90% super-coiled DNA
- Homogeneity of batches
- Possibility of secure storage for recurring plasmid productions
- Adaptation of the process in case of low copy plasmids or recombinations.
- Bacterial transformation
- Adjustment to the desired DNA concentration
- Aliquoting
- Sequencing control (single or double strand, insert or entire plasmid)
- Measurement of the supercoiled rate
- Determination of bacterial endotoxins (LAL / Limulus Amebocyte Lysate test)
- Sterility test
- Sequencing
- Others: contact us
- Transfection / Co-transfection in mammalian cells
- Production of recombinant proteins and recombinant antibodies
- In vivo studies (injection in animals)
- Preclinical studies
- Toxicology studies
- Research & Development applications
High Quality Grade (“GMP-like” grade) plasmid production
Production of superior quality plasmids, suitable for clinical applications, as raw materials in cell and gene therapy programs (AAV, lentivirus, retrovirus, therapeutic mRNA …)
- Complete traceability and specific documentation for each project
- Constitution of a bacterial cell bank for each plasmid
- Storage of the bacterial bank in a secure area
- Each plasmid is produced independently (1 plasmid / series) in a dedicated room.
- Large-scale culture performed without antibiotics
- The production process is carried out “animal free” – without substances of animal origin
- Additional steps are applied to guarantee maximum product purity (quality standards compatible with raw materials for GMP grade production)
- Quality controls are adapted to the custom specifications and to the regulation (% supercoiled DNA, bacterial DNA, residual host proteins, bacterial RNA, Endotoxins… list of QCs)
- Option : Supply of linearized plasmid DNA, for IVT applications (mRNA preparations)
GMP grade plasmid production
Opening of our GMP grade pDNA production facilities in Q3 2023, to complement our current DNA plasmid production capacities in R&D and “High Quality” grades, to meet growing new cell and gene therapy market demands.
- More than 1,200 m2 of GMP grade laboratories
- Several independent production suites
- GMP scale: up to 20 g of highly purified pDNA
Contract services
Dedicated to excellence in molecular biology, RD-Biotech provides cutting-edge solutions precisely tailored to meet the requirements of customers. Our services encompass various expertise areas, ranging from the construction of plasmid vectors — crucial elements in DNA and RNA-based therapies or recombinant protein expression vectors — to high quality RNA or DNA preparation, or even monoclonal antibody sequencing for optimal clone preservation and recombinants expression, …
Why choose our services:
- Customized Service: tailored to your specific needs, ensuring a personalized approach for each project.
- Cutting-edge Technology: continually investment to guarantee high-quality results.
- Expert Team: a team of experienced scientists to guide you at every stage, from understanding your needs to delivering the final product and analysis report.
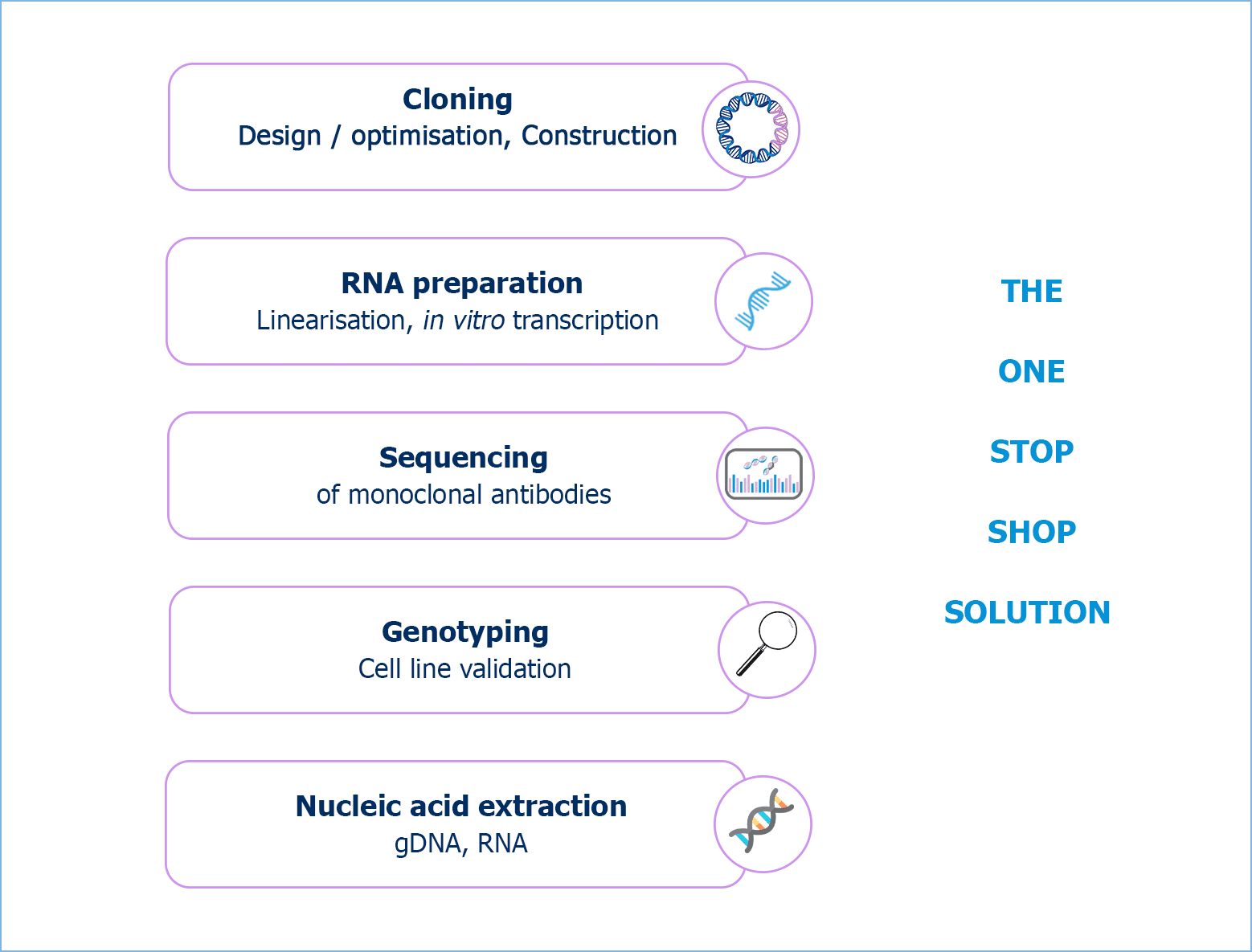
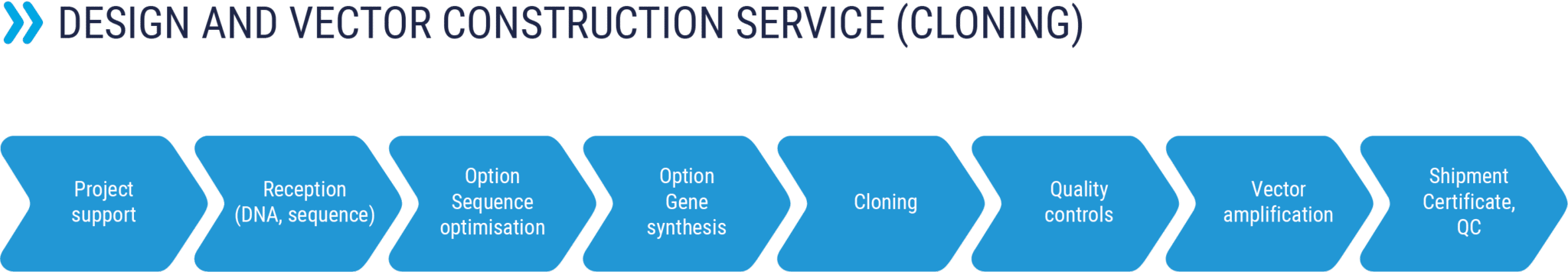
Vector cloning
RD-Biotech can take charge of the construction or modification of your DNA vectors, whatever their application, through a personalized service in order to meet the exact needs of our customers.
Our team of molecular biology specialists can work with you to define the best cloning strategy according to your specifications:
- In silico study
- Choice and design of the nucleic acid sequence of interest
- Optimization of the sequence if required
- Sequence synthesis (PCR, gene synthesis)
- Choice of cloning vector (tag addition, promoter sequences, insertion of specific cleavage sites, co-expression / or not …)
- Choice of cloning technology: Homologous recombination, Enzymatic restriction / ligation, Mutagenesis, Golden Gate…
- Nucleotide or protein sequence (with or without optimization)
- Insert in a shuttle vector
- Cloning according to an optimized strategy
- Plasmid production: from a few µg of DNA up to large scale production
- Quality controls:
- Quantification by spectrophotometry
- Control of restriction profile by agarose gel electrophoresis
- Sequencing (full insert)
A dedicated project manager for each project, in order to ensure constant monitoring and to provide responsiveness as to the progress of the cloning project.
Each project is made up of successive stages, each stage carried out can be validated by the customer before moving on to the next stage (GO / NO GO), for more flexibility and security.
Confidentiality guaranteed.
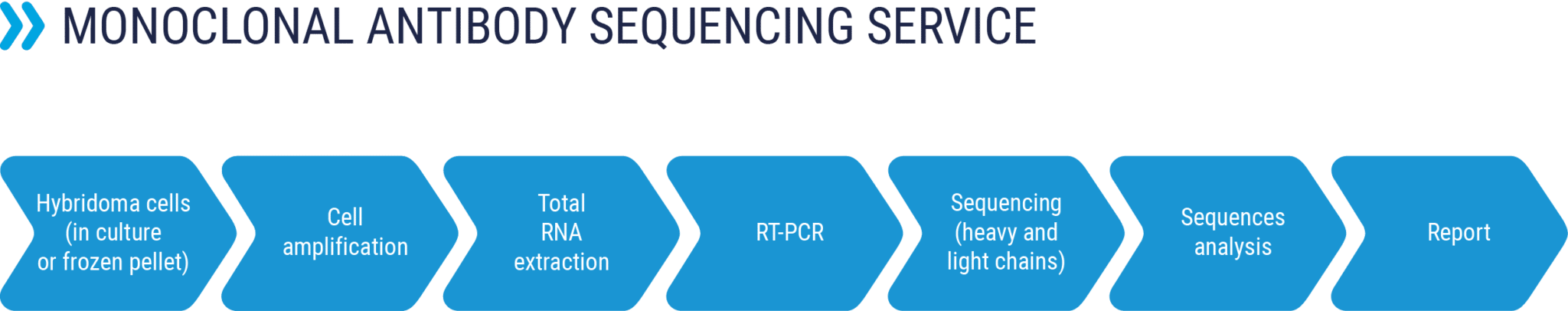
Sequencing of monoclonal antibodies
RD-Biotech offers a fast and reliable sequencing service of your monoclonal antibodies in order to:
- The safeguard and securing of the sequence of your antibody of interest
- Determination of the nucleotide sequences of the heavy and light variable chains in order to produce the antibody in recombinant form
- Starting material: hybridoma cells (in culture or frozen pellet) or dry cell pellet
- Sequencing of variable regions of heavy and light chains or complete sequencing of the antibody
- Results in less than 2 weeks
- Price discounted as part of a complete project: Generation of hybridomas – Construction of the vector and development of recombinants (Backbone IgG murine, human, guinea pig, rat … or other
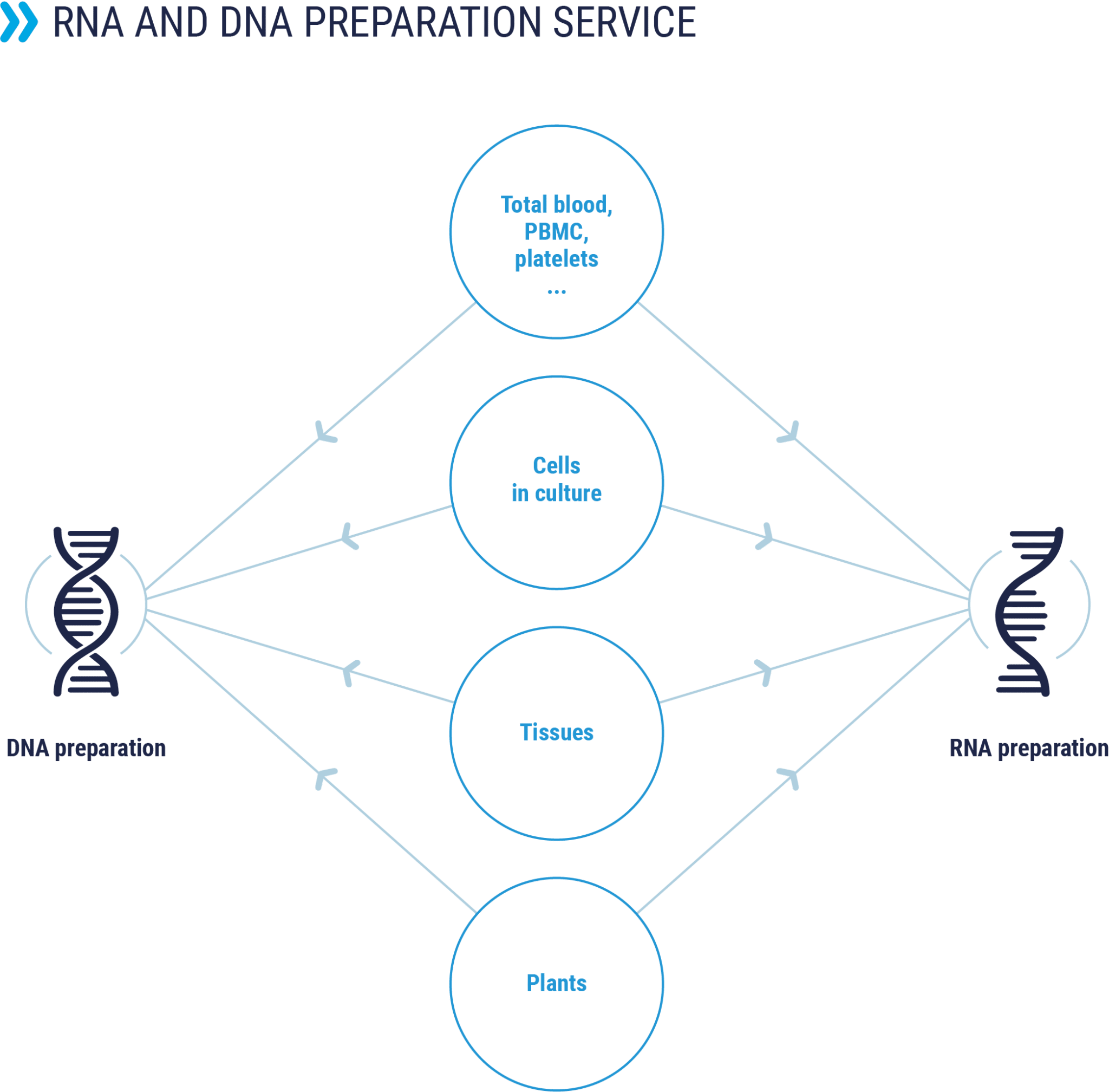
Nucleic acid extraction
Preparation of RNA and genomic DNA
Preparation and purification of RNA and genomic DNA from different biological samples (blood, cell pellets, biopsies, etc.) for research applications or preclinical studies.
The procedure for extraction, purification and control of total RNA and DNA is optimized according to the starting biological material.
- Extraction and purification platform dedicated to the preparation of total RNA and DNA
- Large production capacity: preparation by series of 50 to 200 samples
- Possibility of preserving RNA and DNA preparations (secure guarding) for later uses (Reverse transcription, PCR, Cloning, etc.)
Quality controls:
- RNA and DNA quantification by spectrophotometry
Controls of RNA integrity on chips
Controls of DNA and RNA on agarose gel
Other controls – (contact us)
Nucleic acid amplification: RT-PCR qPCR
RD-Biotech can respond to various issues related to the quantification of genes, the study of gene expression, the analysis of the expression of different variants of a gene, the identification of specific sequences …
- Development of operating conditions or application of a validated procedure
- Amplifications by PCR
- PCR products control by sequencing – if requested
- Analysis of results and delivery of a complete report
- Possibility of preparing samples before analysis
- Development of operating conditions or application of a validated procedure
- Absolute quantification and relative quantification
- SyberGreen® or TaqMan® procedure
- Analysis of results and delivery of a complete report
- Possibility of preparing samples before analysis
Cell line validation / Genotyping
- Control of transgenic sequences
- Measurement of the expression levels of target genes by qPCR
- SyberGreen® or TaqMan® procedure
- Absolute quantification and relative quantification
A service adapted to your needs:
- Preparation of genetic material
- Development of operating conditions or application of validated procedure
- Detailed analysis report
RNA preparation by in vitro transcription
In vitro transcription platform for the generation of RNAs, from your plasmids or your PCR products
- Sense or antisense transcripts
- Labelled or unlabelled transcripts
- Capped and / or poly-adenylated transcripts
- Enzymatic digestion
- Pilote and large scale linearisation
- Purification and quality controls
- Capping
- Modified nucleotides incorporation
- Poly-adenylation – polyA tail or post-transcriptionnal process (enzymatic)
- Quantity: from some µg up to batches of 5 -10 mg
Other services
Our sales team as well as our scientific and technical experts are at your disposal to offer you the service adapted to your needs.