RD-BIOTECH completes its biomanufacturing offer with a new production unit for DNA plasmids, raw materials for the pharmaceutical industry (GMP grade – Good Manufacturing Practices).
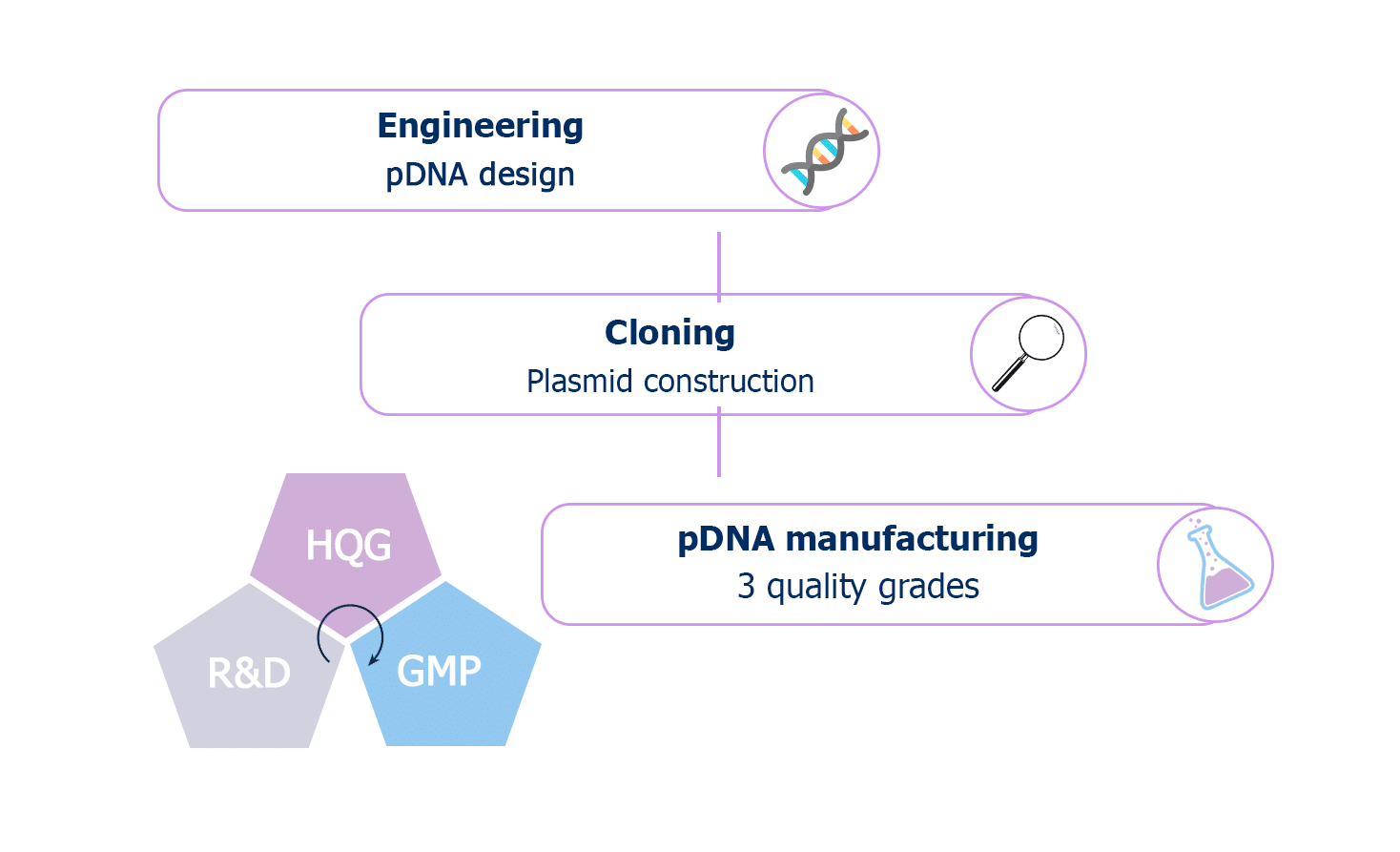
With this new service offering, RD-Biotech is now able to provide a comprehensive service, from plasmid engineering to production, tailored to the quality standards required for its clients’ applications.
Why choose RD-Biotech?
One stop shop partner for your discovery & manufacturing needs
Over 20 years of experience in molecular biology & biomanufacturing
Complete offer from early discovery (pDNA cloning) to commercial manufacturing (GMP grade)
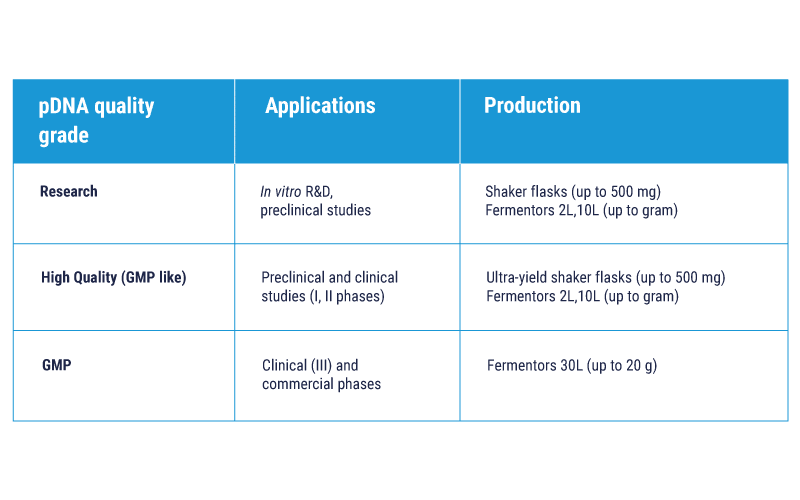
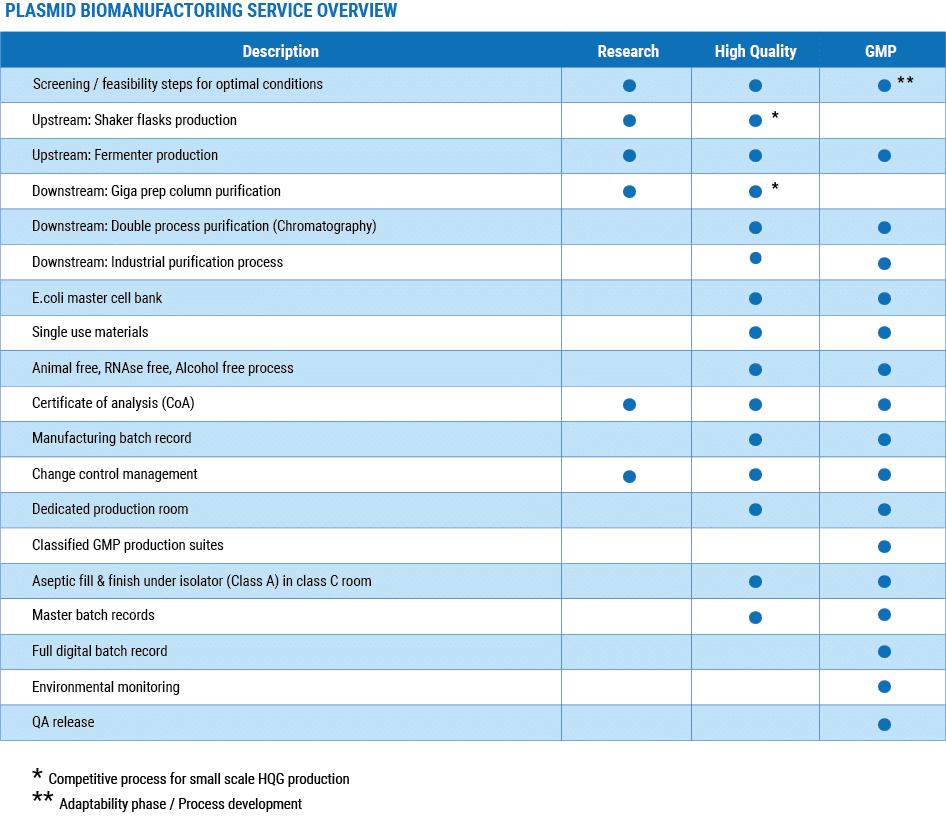
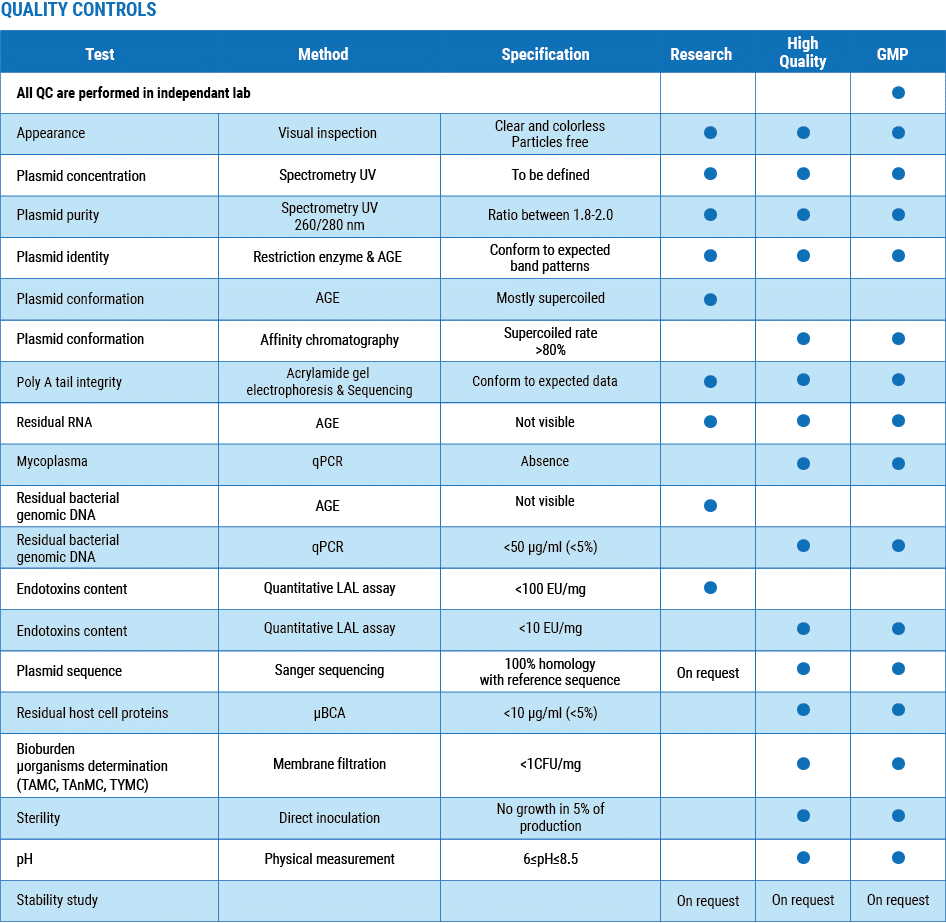
Three quality grades to meet each phase of your development
Scalable process of pDNA production from several mg up to 20 g
Cost effective & adaptable process to meet your requirements
pDNA as starting material: Gene therapy, mRNA vaccine, DNA based therapy, Viral vectors, Cell therapy, CAR-T cells,…
Choose the RD-Biotech quality in GMP-grade plasmid DNA production:To support our customers from R&D phases to commercial production stages of starting materials for cell & gene therapies
To find out more and book your slot:
Winner of the France Relance 2021 prize
RD-Biotech saw the project to build its “GMP plasmids” production unit selected by the French government.